Prof. Jacek Jemielity, Dr. Joanna Kowalska and Dr. Anaïs Depaix from UW, in collaboration with scientists from the University of California, San Francisco, demonstrated how enzyme activity in intracellular condensates contributes to mRNA degradation. The findings will enable further development of mRNA-based therapies. The researchers described the results of their work in the journal “Nature Chemical Biology”.
mRNA molecules have at their 5′ end a unique structure called a cap, which plays an essential role in protein biosynthesis. The process of cap removal is a step that precedes the degradation of messenger RNA. It occurs in protein condensates (so-called P-bodies), which contain nucleic acid and Dcp1/Dcp2 enzyme responsible for mRNA degradation.
Researchers from the University of Warsaw: Prof. Jacek Jemielity from the Center for New Technologies of the University of Warsaw, Dr. Joanna Kowalska and Dr. Anaïs Depaix from the Faculty of Physics of the University of Warsaw, in collaboration with researchers from the University of California, San Francisco, have shown how the activity of Dcp1/Dcp2 enzyme can be “switched on” or “switched off” in condensates. The team’s findings were published in the paper “Biomolecular condensates amplify mRNA decapping by biasing enzyme conformation” in the journal “Nature Chemical Biology”.
“In simple terms, condensates resemble oil suspended in water. They are a separate liquid substance, different from the surrounding cytoplasm. They are responsible for separating various biochemical processes taking place in cells,” says Prof. Jacek Jemielity from the Center of New Technologies of the University of Warsaw.
Activity of enzymes
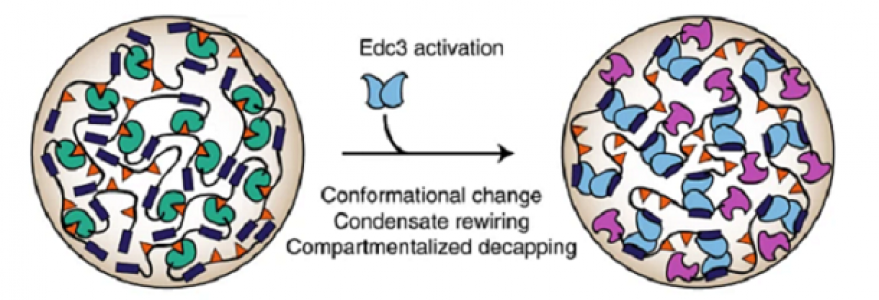
The researchers discovered that Dcp1/Dcp2 enzyme activity is regulated by protein-protein interactions that lead to liquid-liquid phase separation and condensate formation. Separation of the Dcp1/Dcp2 enzyme fixes it and inhibits cap removal from mRNA. The addition of another protein, Edc3, causes enzyme changes that lead to the removal of the cap structure in the mRNA stored in the condensates and consequently allows mRNA degradation.
The results indicate that the structures where phase separation occurs are sites of very precise enzyme activation/deactivation. These findings contribute to our understanding of how sometimes seemingly opposing biochemical processes can take place in protein condensates that have not yet been elucidated.
mRNA therapies
Obtaining the results of the research described in the article was possible thanks to the development by a team of researchers from the UW of a method for labelling of the structure of mRNA with fluorescent tags. The results of the study will help to better understand the changes occurring in cells and will enable the development of further mRNA therapies.
“The processes of mRNA degradation, in which Dcp1/Dcp2 enzymeparticipates, have a direct impact on the intracellular stability of mRNA-based therapeutics and vaccines. To enable observation of mRNA degradation process in condensates, we created RNA molecules specifically labeled (glowing) with a different color at each end. In the future, this method may also be used to track the degradation of mRNA-based therapeutics, and, consequently, to search for factors that slow down this process,” explains Dr. Joanna Kowalska from the Faculty of Physics, University of Warsaw.
The text of the article can be found on the website of the journal “Nature Chemical Biology”.