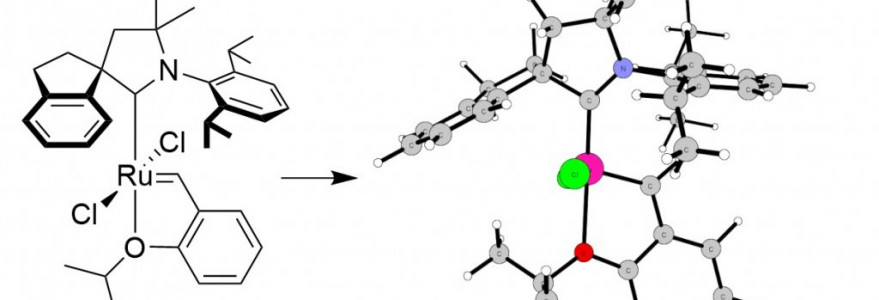
Prof. Bartosz Trzaskowski and Dr Juan Pablo Martinez from the UW’s Centre of New Technologies contributed to the creation of a new class of ruthenium catalysts important for ethenolysis – one of the most efficient processes by which compounds generated in petroleum processing can be obtained from vegetable oils. The scientific paper, with the contribution of the UW researchers, was published in the Journal of the American Chemical Society.
Chemical catalysis is the phenomenon of accelerating a chemical reaction resulting from the participation of an additional substance called a catalyst. Catalytic reactions are preferable in environmentally friendly green chemistry because of the reduced waste generated and power as well as time consumed.
Nowadays, the production of the most industrially important chemicals involves catalysis and similarly most biochemically significant processes are also catalysed.
“From a chemical point of view, a catalyst lowers a free energy barrier of a reaction, meaning that less free energy is required for a substrate to reach its transition state. Catalysed reactions have lower activation energy (rate-limiting free energy of activation) than the corresponding non-catalysed reaction, resulting in a higher reaction rate at the same temperature and for the same reactant concentrations,” says Prof. Bartosz Trzaskowski from the UW’s Centre of New Technologies, who, together with Dr Juan Pablo Martinez from the same unit, co-authored the publication in the Journal of the American Chemical Society.
Scientists indicate that the mechanism of the catalysed reaction is complex, and catalysis itself is a multi-step process. Each chemical reaction requires a unique catalyst. In their research, scientists aim to obtain the most stable substance possible, capable of catalysing a chosen reaction for as long as possible.
“The most important parameter characterizing a catalyst is the turnover number, which is the number of substrate molecules that are converted into the product by the catalyst before it deactivates. The higher it is, the more efficient the catalyst,” Prof. Trzaskowski explains.
Emerging green technology
The scientists carrying out this project wanted to develop new, efficient catalysts for ethenolysis, a chemical process in which internal olefins (compounds with at least one C=C double bond) are degraded using ethylene as a reagent. The reaction is a unique case of metathesis. It has been named “emerging green technology” by the Royal Academy of Science during the 2005 Nobel Prize ceremony. Research groups quickly adopted it as a basic strategy for the synthesis of carbon-carbon bonds. The ability of this method for the selective substitution of atoms between two molecules allows the generation of chemical systems with the desired properties.
Ethenolysis is one of the most efficient processes by which compounds usually generated in petroleum processing can be obtained from vegetable oils.
As the researchers point out, a new class of ruthenium catalysts, designed and synthesized in collaboration with Prof. Trzaskowski and Dr Martinez, showed excellent properties in catalysing ethenolysis reactions.
“In model ethenolysis reactions, these catalyst showed turnover number above 2.5 million, several times higher than previously known catalysts of ethenolysis, and at very low concentrations (more than a million times lower than the concentration of substrates). In specific applications, e.g. in the ethenolysis of methyl esters obtained from sunflower oil and rapeseed oil, which are cheap and available on an industrial scale, an impressive turnover number of about 1 million was obtained at similarly low catalyst concentrations,” Dr Juan Pablo Martinez emphasised.