A team of scientists from the Centre of New Technologies of the University of Warsaw, and the California Institute of Technology presented two-dimensional metal-organic frameworks with significantly improved methane and ethylene formation yield. Research findings were published in the Journal of the American Chemical Society.
Electrocatalytic conversion of carbon dioxide into valuable chemicals such as methane, ethylene, and ethanol is a significant process regarding environment-friendly energy production. Scientific research proves that metallic copper remains the best electrocatalyst. However, its efficiency is limited. Scientists are looking for other solutions that enable cheaper and more green chemical reactions.
A team of researchers from the University of Warsaw and the California Institute of Technology have proposed to use two-dimensional metal–organic frameworks in this regard. The frameworks are the materials formed from metal ions or inorganic clusters connected by rigid organic connectors.
“Two-dimensional metal–organic frameworks meet both of these criteria and have attracted great interest because of their ease of synthesis and their catalytic and semiconducting properties. The appeal of the two-dimensional metal–organic frameworks lies in their modular structure, large material surface area and high porosity. Thanks to their characteristics, they can be applied in the separation and storage of gas, mixture separation, or transport of medicines,” explains Prof. Silvio Osella from the Chemical and Biological Systems Simulation Laboratory of the Centre of New Technologies (CeNT), the University of Warsaw.
Enhancing ethylene production
The UW researcher points out that the common electrocatalytic process to obtain ethylene from carbon dioxide is multi-step. The key process, however, is the one, in which the CO-CO molecule is formed.
“The energy required to obtain such a dimer is often very high, requiring an elevated temperature or other harsh conditions for the reaction to proceed. Therefore, the goal of many scientific groups is to develop new chemical systems that will render this step fast, making the entire process both time and cost-effective,” Prof. Bartosz Trzaskowski from the CeNT says.
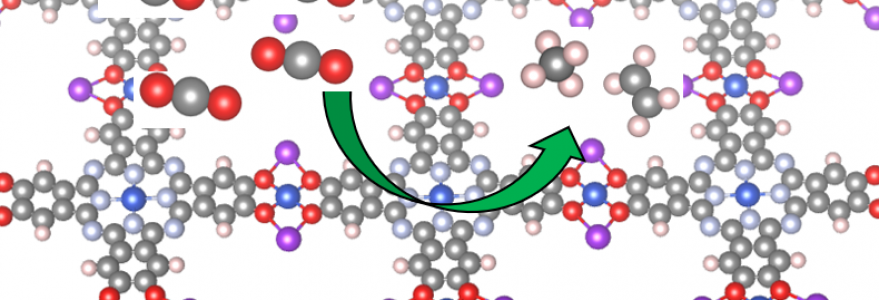
Due to the confinement of the substrate within such low-dimensional materials, new fascinating properties arise that can be exploited for the increase in the production yield for many types of important chemical reactions. “In this study in the Journal of the American Chemistry Society, we explore how a particular class of two-dimensional metal–organic frameworks based on a phthalocyanine core provides the reactive centre for the production of ethylene and methane,” Prof. Osella indicates.
New reaction mechanisms
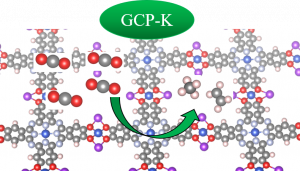
The publication’s author, who was a lead author and a contractor, explains that scientists have examined the catalytic reaction mechanism using the Grand Canonical Potential Kinetics (GCP-K) or Grand Canonical Quantum Mechanics (GC-QM) computational methodology
These new and accurate computational methods, developed at the California Institute of Technology, allow one to estimate reaction rates at a constant applied potential, which can be compared directly with experimental data, making the prediction power of computation much more accurate.
“Thanks to these methods, we have explained the reaction mechanism underlying the formation of methane and ethylene on the studied two-dimensional metal–organic frameworks, with a production yield of up to 50% for ethylene and 25% for methane – one of the highest reported for two-dimensional materials,” Prof. Osella says. “We have also shown that the key reaction step is different from the usual CO-CO dimerization step observed on copper metal surfaces, requiring much less energy. As a result, the two-dimensional metal–organic frameworks behave like a single atom catalyst,” he adds.
In the future, the methodology can be used to design new two-dimensional metal–organic frameworks or other materials that will allow more efficient conversion of CO2 to methane and ethylene or other fuels and chemical raw materials. Such artificial conversion of carbon dioxide is essential both to reduce its emissions and to pursue sustainable development, threatened by fossil fuel depletion and their non-renewability.