Researchers from the Centre of New Technologies together with their colleagues from the United States and Slovenia, have discovered new unique type of nanotubes.
Carbon nanotubes, made from carbon atoms arranged in hexagonsare, are probably the best known nanotubes. They are linked together in a honeycomb pattern, similarly as in graphene. The key difference between graphene and a nanotube is that graphene sheets are flat, while in a nanotube they are bent and „sewed“ by chemical bonds into a single roll. Nanotubes may be filled inside, or empty.
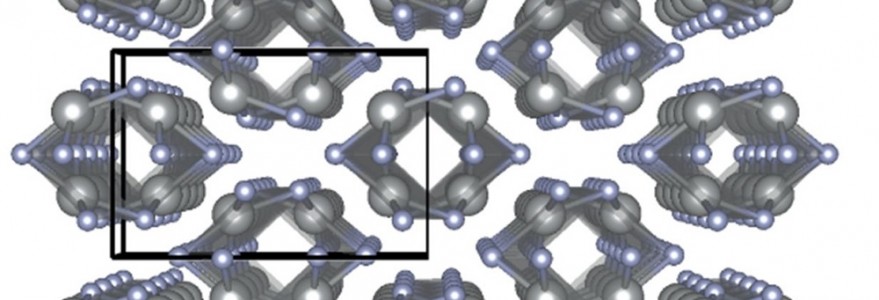
The team from the University of Warsaw led by Prof. Wojciech Grochala working closely with Dr. Zoran Mazej at the Slovenian Jožef Stefan Institute in Ljubljana and Dr. Viktor V. Struzhkin at Carnegie Institution of Washington, USA, discovered an entirely new type of nanotubes. They contain only silver and fluorine, and – unlike carbon nanotubes – they are made of squares rather than hexagons. If a nanotube would be cut open it would resemble chessboard rather than honeycomb.
– We wanted to squeeze the compound containing silver and fluorine, AgF2, to see whether its atomic layers will flatten out or pucker. Instead, they rolled into a new kind of nanotubes! It was an unexpected but fantastic discovery – explains the theoretician on the Warsaw team, Dr. Mariana Derzsi.
– For a very long time we could not understand the experimental data – says Adam Grzelak, the PhD student. – Suddenly, theoretical calculations helped us to realize that peculiar nanotubes have formed. And, in a blink of an eye, all data started to make sense – he adds.
It is uncertain what the new nanotubes might be good for. One clue comes from the presence of silver in their structure. – The silver ions, Ag2+, present in AgF2, show interesting chemical, electronic, magnetic and optical properties. AgF2 is produced in ton amounts worldwide and used for fluorination reactions. So one might expect that nanotubes could be used in the future as catalysts of chemical reactions, or for spintronics and molecular electronics applications – explains Prof. Wojciech Grochala. – However, AgF2 is extremely reactive, and preparation of nanotubes requires the use of high pressure, so the prospect for applications is not certain. We must first learn how to prepare nanotubes at ambient pressure – he adds.
The research was published in Dalton Transactions and highlighted by internet chemistry portal chemistryviews First Metal Fluoride Nanowire. Further details were published in a separate contribution in Inorganic Chemistry.