Researchers from the University of Warsaw together with their colleagues from the University of Freiburg have discovered a new mechanism of protein synthesis regulation. The study was published in Nature Communications.
Mitochondria are cellular organelles that are frequently named powerhouses of the cell for their important role in energy conversion. Mitochondrial function depends on the cellular protein synthesis machinery since 99% of mitochondrial proteins are nuclear encoded and need to be synthesized outside of mitochondria and imported into mitochondria. Dr. Ulrike Topf from the group of Prof. Agnieszka Chacińska, currently at the Centre of New Technologies, the University of Warsaw (relocated from the International Institute of Molecular and Cell Biology in Warsaw) sought to understand how mitochondria can communicate their need for newly synthesized proteins coming from outside the organelle.
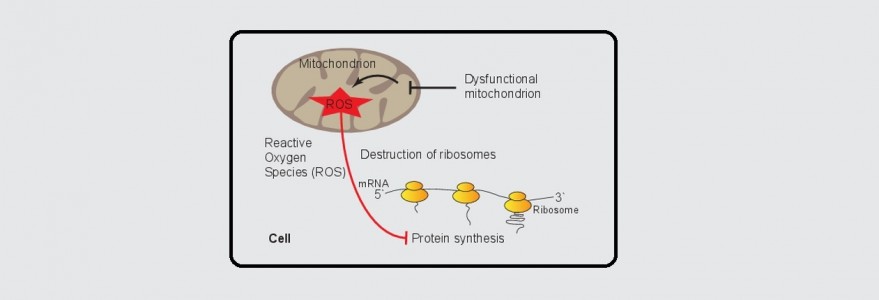
In a collaborative effort with the group of researchers from Germany led by Prof. Bettina Warscheid from the University of Freiburg, we describe a novel mechanism by which dysfunctional mitochondria regulate a key cellular process, which is the protein synthesis. Interestingly, these cellular powerhouses do so by informing protein synthesis machinery about their problems and defects releasing reactive oxygen species (ROS). This information leads to a slowdown in cellular protein synthesis that allows faster recovery and returns to cellular homeostasis. The study has now been published in Nature Communications.
The initial question posed by Chacińska and Topf has been pursued by proteomics experts in Germany, who applied a cutting edge quantitative approach to map redox-active thiols in proteins exposed to mitochondria-derived ROS. The analysis of Dr. Ida Suppanz, among others from the Warscheid group revealed ROS-sensitive sites in several components of the protein synthesis apparatus. Ulrike Topf using sophisticated protein biochemistry and cell biology techniques and a simple yeast Saccharomyces cerevisiae as a model organism, observed that upon increased ROS levels global protein synthesis was attenuated. She was able to find an exciting molecular link, a redox switch, that serves to shutdown protein synthesis in response to mitochondrial stress. Next, Dr. Łukasz Samluk investigated whether this newly discovered regulation is unique for budding yeast. He demonstrated that protein synthesis regulation by defective mitochondria also exists in mammalian, and specifically human, cells. In sum, the scientists discovered a new exciting regulation of protein synthesis that involves a redox switch at the ribosomes.
The finding that mitochondria can signal their current state via mitochondrial-derived ROS and can thereby adjust directly global cytosolic protein synthesis provides important implication in understanding consequences of mitochondrial and protein folding pathologies, such as neurodegeneration.
Research of the Chacinska group was funded by the National Science Centre and Ministry of Higher Education.