In collaboration with Japanese researchers, a team of scientists from the UW carried out the research on riboswitches, mRNA segments, which control the production of proteins in bacteria. The paper devoted to this study was published in “Proceedings of the National Academy of Sciences”.
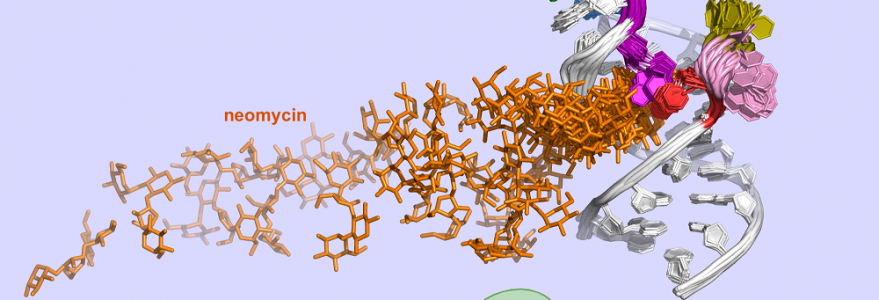
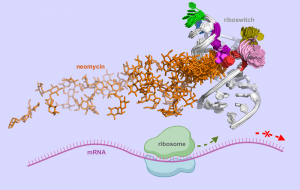
Prof. Joanna Trylska, Piotr Chyży and Marta Kulik from the University of Warsaw performed together with the group of Prof. Yuji Sugita from RIKEN (Rikagaku Kenkyūjo), a research institute in Japan, multidimensional molecular dynamics simulations of neomycin-riboswitch systems. The simulations were conducted using supercomputers at the University of Warsaw (the Centre of New Technologies and Interdisciplinary Centre for Mathematical and Computational Modelling), and the RIKEN Advanced Centre for Computing and Communication.
Riboswitches
Riboswitches are segments of messenger RNAs (mRNAs) located in front of the protein-coding part of the mRNA. They change structure when they bind to specific small molecules, such as metabolites. This structural change of the riboswitch affects whether the protein is made or not. Influencing the state of riboswitches could provide control over the production of specific proteins essential for cell life.
“Naturally occurring riboswitches could be targets for new antibiotics. Designing molecules that block these switches in bacteria could lead to the death of bacterial cells. However, riboswitches are not present in all cells, which is why we are interested in synthetic riboswitches. These can be incorporated into mRNA, for example in mammalian cells, giving us control over the production of particular proteins. Nevertheless, designing riboswitches is challenging because it requires predicting how their structure will change upon binding to a specific molecule. Even if a molecule binds tightly to a riboswitch, it does not always ensure that the riboswitch will function as expected in living cells,” Prof. Joanna Trylska from the UW’s Centre of New Technologies said.
Molecule binding
The researchers investigated a small synthetic riboswitch that binds neomycin. They developed a method and tools to describe the association pathway of neomycin to the riboswitch at an atomistic level of detail. The results of their simulations elucidated the mechanism of functioning of this riboswitch. This insight can help design synthetic riboswitches and molecules that control their dynamics and structure.
“Our work shows how the crucial steps of the interaction of neomycin with the riboswitch can be optimized through mutations to enhance riboswitch regulatory effectiveness in cells. The results highlight key dynamic and structural differences along the binding pathway for the different experimentally reported mutants, unravelling the effects of mutations on the riboswitch activity that cannot be determined solely through experiments,” Prof. Trylska added.
Against bacterial cells
The methodology and tools developed by the scientists can facilitate the drug design process by helping to select the most promising molecule-riboswitch pairs for further experimental validation. The results of the research carried out by the scientists at the UW and RIKEN Institute can accelerate the design of controllable RNA fragments as a potential strategy in the fight against antibiotic resistance.
Piotr Chyży, Marta Kulik, Ai Shinobu, Suyong Re, Yuji Sugita, Joanna Trylska, “Molecular dynamics in multidimensional space explains how mutations affect the association path of neomycin to a riboswitch”, Proceedings of the National Academy of Sciences. The text of the article can be found on the PNAS website.