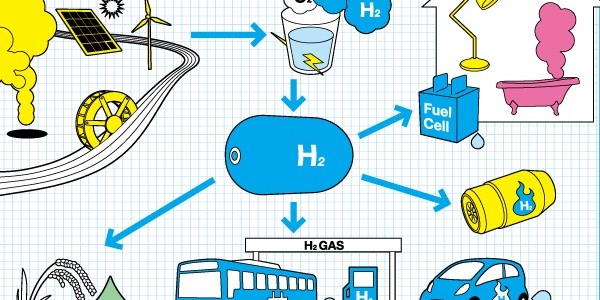
Hydrogen, the most abundant element in the Universe, is considered to be clean and renewable fuel of the future, which could permit achieving sustainable economy using exclusively sunlight.
However, free (elemental) hydrogen is not found on our planet, and it must be obtained from either water or hydrocarbons. Moreover, H2 is a gas at ambient conditions and it must be either compressed or liquefied to be stored as a fuel tank for mobile applications. This is why there is an intense ongoing research worldwide on storing hydrogen in the solid state, with the target hydrogen content of 7.5–9.0% by weight.
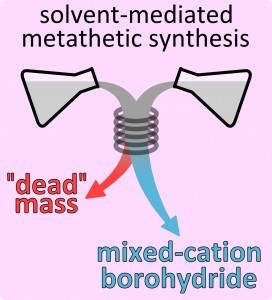
Metal borohydrides are chemical compounds which consist of metal(s), boron and hydrogen, and they usually contain large amounts of the latter. For example, lithium borohydride (LiBH4) contains as much as 18.4% of hydrogen by weight. This and other – more complex – metal borohydrides are now considered as the most promising candidates for hydrogen storage materials in the solid state. The problem, however, exists, that current methods of synthesis of the complex hydrides containing two different metals yield products, which are severly contaminated with LiCl by-product. This so called “dead weight” is inseparable from metal borohydride by common chemical or physical means, and it severely decreases the practical hydrogen content of the product. Moreover, the dry mechanochemical method, which is typically used for synthesis, is not easily-scalable and even potentially dangerous, given the substantial reactivity of borohydride towards atmospheric air.
In their short communication the team composed of researchers from the Center of New Technologies (CeNT) and students from the Faculty of Physics, University of Warsaw (Poland) described the new method which bypasses all existing problems simultaneously. The scientists have used a two-step metathesis reaction in organic solvents, which permits synthesis of metal borohydride while “dead mass” (LiCl or NaCl) is separated by filtration in the first step. They have successfully synthesized light weight lithium/zinc and sodium/zinc borohydrides using their new approach as well as extend the range of known compounds in the family of alkali metal/yttrium borohydrides. Synthesis of many more systems based on Mg, Sc, Mn and other transition metals, is now carried out at CeNT.
The wet chemistry metathetic method is scalable, it uses low-boiling organic solvents and delivers high-purity product, the features which render it energy- and materials-effective.
The new technology has been protected by Polish and international (PCT) patent applications and it is now sought to be commercialized.